Abstract
Background: Gilteritinib, a highly selective, potent FLT3/AXL inhibitor, showed antileukemic activity with favorable tolerability in a phase 1/2 trial of patients (pts) with FLT3mut+ relapsed/refractory AML (Perl AE, et al. Lancet Oncol. 2017). Gilteritinib plus azacitidine (AZA) synergistically induced apoptosis and inhibited growth in the MV4-11 cell line and in MV4-11 tumors (Ueno Y, et al. Blood. 2016). The safety, tolerability, and efficacy of gilteritinib alone, gilteritinib plus AZA, or AZA alone is being evaluated in pts with newly diagnosed FLT3mut+ AML ineligible for intensive induction chemotherapy; this abstract presents data from the Safety Cohort which evaluated only gilteritinib plus AZA.
Methods: Adults with newly diagnosed FLT3mut+ (FLT3-ITD or FLT3-TKD) AML are being enrolled in this ongoing clinical trial (NCT02752035). Prior to initiation of the randomized portion of the study, the appropriate gilteritinib dose for combination therapy was assessed in a Safety Cohort. Patients enrolled in this cohort received escalating doses of oral gilteritinib (80 or 120 mg/day) on Days 1-28 in combination with subcutaneous or intravenous AZA (75 mg/m2/day) on Days 1-7. Observations of dose-limiting toxicities (DLTs) were collected through Cycle 1; treatment was continued in 28-day cycles until lack of clinical benefit or unacceptable toxicity. Safety and tolerability were the primary endpoints of the Safety Cohort; antileukemic response rates (ie, complete remission [CR], CR with incomplete platelet recovery [CRp], CR with incomplete hematological recovery [CRi], partial remission [PR], and overall response rate [ORR]) were also assessed.
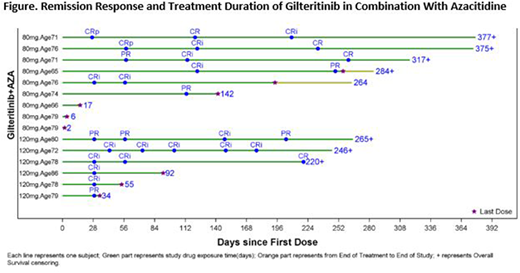
Results: A total of 15 adult pts (median age, 76 [range: 65-86]) were enrolled into the Safety Cohort (n=9, 80 mg gilteritinib; n=6, 120 mg gilteritinib); 14 pts were FLT3mut+ (ITD alone, n=10; TKD alone, n=3; ITD and TKD, n=1) and 1 pt had no FLT3 mutation. As of 25 June 2018, more than half (n=8/15; 56%, Figure) of the pts had a treatment duration of >6 mo, while 9 pts discontinued treatment (death, n=4; relapse; adverse event [AE]; physician decision; sponsor decision; subject withdrawal, n=1 each) and 6 pts remained on treatment. During the DLT observation period, 1 DLT (tumor lysis syndrome) was observed in a pt who received 80 mg gilteritinib plus AZA; no DLTs were reported in pts who received 120 mg gilteritinib plus AZA. One or more AEs were seen in all 15 pts; 12 (80%) experienced AEs considered at least possibly related to treatment. Adverse events occurring in ≥25% of pts were anemia (n=7), febrile neutropenia and nausea (n=6 each), increased ALT and AST, constipation, diarrhea, neutropenia, thrombocytopenia, and pyrexia (n=5 each), and decreased appetite, fatigue, increased blood creatinine, and hypocalcemia (n=4 each). Grade ≥3 AEs occurring in ≥25% of pts were febrile neutropenia (n=6), anemia and neutropenia (n=5 each), and thrombocytopenia (n=4). Serious AEs occurring in >2 pts were febrile neutropenia (n=5), and anemia and pyrexia (n=3 each). Of the 8 pts with fatal AEs, none of which were related to treatment, 3 occurred early in treatment: septic shock (Day 2), respiratory failure (Day 6), and cerebral hemorrhage in the setting of acute kidney injury and uremia (Day 17). None of the 13 pts with post-baseline lab data had any potentially clinically significant AST/ALT (>3 X ULN) and total bilirubin (>2 X ULN) values; none of the pts had a maximum post-baseline QTcF interval >500 msec.
Across the Safety Cohort, a composite complete remission rate of 67% (n=10/15) was observed; 4 pts achieved a best overall response of CR and 6 achieved CRi (Figure). Two additional pts (13%) achieved a best overall response of PR, giving an ORR of 80%.
One DLT from the 11 DLT evaluable pts (defined as pts who experienced a DLT, or in the absence of DLT, received at least 23 of 28 doses of gilteritinib and at least 5 of 7 doses of AZA during the DLT observation period) informed the decision to proceed to the randomized portion at a dose of 120 mg gilteritinib for the combination treatment arm.
Conclusions: Gilteritinib and AZA were generally well tolerated with no unexpected AEs. This combination therapy induced antileukemic responses in newly diagnosed FLT3mut+ AML pts unfit to receive standard induction chemotherapy. Based on these results, pts are being enrolled into the randomized portion of the study with a dose of 120 mg gilteritinib for the combination treatment arm.
Wang:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Jazz: Speakers Bureau; Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy. Laribi:Amgen: Other: Personal fees; Takeda: Other: Grant and personal fees; Sandoz: Other: Grant; Novartis: Other: Grant and personal fees; Teva: Other: Grant; Gilead: Other: Personal fees; Hospira: Other: Grant; Roche: Other: Grant. Montesinos:Daiichi Sankyo: Consultancy, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Liu:Astellas Pharma: Employment. Rich:Astellas Pharma: Employment. Bahceci:Astellas Pharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal